What is Silane Coupling Agent?
Silane coupling agents might sound complex, but their role is simple yet powerful: they act as molecular "bridges" to bond materials that normally don’t stick together. Imagine trying to glue plastic to glass—without a middleman, they’d struggle to adhere. Silane coupling agents solve this problem by creating strong, durable connections between organic materials (like plastics, rubbers, or resins) and inorganic materials (such as glass, metals, or minerals).
These agents work through a clever two-part structure:
-
Hydrolyzable groups (X): These react with water to form reactive "silanols" that latch onto inorganic surfaces.
-
Organic functional groups (R): These non-reactive tails bond with organic materials like polymers.
When applied, silanes first "activate" by reacting with moisture (even from the air!) to form silanols. These then cling to inorganic surfaces (e.g., glass fibers in composites) and form strong silicon-oxygen bonds. Meanwhile, their organic tails seamlessly integrate with resins or plastics. The result? A seamless hybrid material with improved strength, durability, and performance.
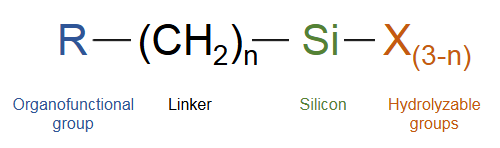
How Do Silane Coupling Agents Work?
Let’s break down the science into everyday terms. Think of silane coupling agents as matchmakers for materials.
Here’s their four-step process:
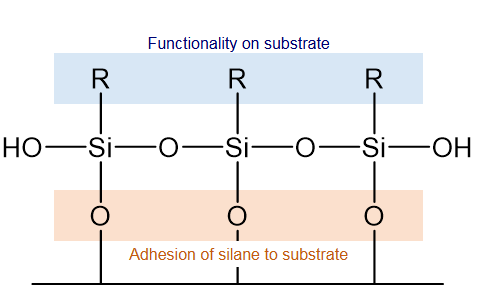
Hydrolysis: When silanes meet water (from the air, surfaces, or added moisture), their "X" groups transform into sticky silanol (-Si-OH) molecules.
Condensation: These silanols bond with inorganic surfaces (like glass or metal oxides) to form tough siloxane (-Si-O-Si-) networks.
Orientation: The molecules arrange themselves horizontally on the surface, optimizing contact.
Curing: Heat or drying locks the bonds in place, creating a stable bridge.
For example, in fiberglass-reinforced plastics, silanes bond glass fibers to the resin matrix. Without them, the fibers would slip under stress, weakening the composite. With silanes, the bond withstands heat, moisture, and mechanical strain—key for products like car parts or wind turbine blades.
Applications of Silane Coupling Agents
Silane coupling agents are unsung heroes in countless industries.
Here’s where they shine:
Composite Materials: Boost strength in fiberglass, carbon fiber, and mineral-filled plastics (e.g., automotive parts, aerospace components).
Coatings & Adhesives: Improve paint adhesion to metals or ceramics while resisting corrosion.
Electronics: Protect microchips by enhancing resin-to-silicon bonds in semiconductor packaging.
Dental & Medical: Strengthen tooth fillings (bonding resins to glass ionomers) and improve biocompatibility in implants.
Textiles: Waterproof fabrics by bonding silicone coatings to fibers.
A real-world example? In tires, silanes bond silica fillers to rubber, reducing rolling resistance (improving fuel efficiency) while enhancing grip.

What to Consider When Selecting a Silane Coupling Agent
Choosing the right silane is critical for success. Key factors include:
Substrate Compatibility:
- Inorganic Surface: Match the hydrolyzable group (X) to the substrate. For example:
-
-
Alkoxy groups (e.g., methoxy): Ideal for glass, silica, or metal oxides.
-
Chlorosilanes: Work well with metals but require careful handling.
-
-
- Organic Phase: Align the R group with your polymer. Amino silanes suit epoxy resins, while vinyl silanes bond with polyethylene.
Hydrolysis Conditions:
- Low humidity? Use pre-hydrolyzed silanes to skip moisture dependency.
- For vapor-phase deposition (e.g., semiconductor coatings), cyclic azasilanes deliver superior monolayer coverage.
Environmental Stability:
- In humid environments, choose silanes that resist re-hydrolysis (e.g., phenyl-substituted silanes).
- For high-temperature applications, ensure the R group won’t degrade (e.g., epoxy-functional silanes withstand >200°C).
Regulatory Compliance:
- In food-contact or medical applications, opt for FDA-approved silanes like 3-aminopropyltriethoxysilane.
By balancing these factors, engineers can tailor silanes to solve specific bonding challenges—whether creating lightweight aircraft composites or durable dental restorations.
Here in Beihua, we have 3 different silane coupling agents, SI69-50, Silane Companion Powder, Silane Companion Liquid.
If you do not want silane coupling agents, take a look at our carbon black IMLV/N330, which is also a nice coupling agent to improve the elastic modulus and tensile strength of rubber.
Conclusion
Silane coupling agents are tiny molecules with an outsized impact on modern materials. By mastering their chemistry and applications, industries can design stronger, lighter, and more sustainable products. From everyday plastics to cutting-edge tech, these agents prove that even the smallest bonds can drive big innovations.
 vaskokudrickrk136@gmail.com
vaskokudrickrk136@gmail.com Jiaxing Beihua Polymer Auxiliaries Co., Ltd. / Shanghai Crystal Wells Chemical New Materials Co., Ltd.
Jiaxing Beihua Polymer Auxiliaries Co., Ltd. / Shanghai Crystal Wells Chemical New Materials Co., Ltd.



































